Please welcome back Brandon Moyer for another excellent post. 
We carry hundreds of different species of fish and inverts here at That Fish Place, That Pet Place that come from all around the world. There are, however, certain species that are no longer available to us by act of law. Their release into the wild and the lifestyles and behaviors they exhibit has earned them the title of invasive species. This blog is the first in a series of popular invasive pet species accounts. One of these is commonly inquired about here at That Fish Place and is notorious worldwide.
The Northern Snakehead, Channa argus, is one species of fish that has been introduced into non-native waters where it has thrived and disrupted its new habitat. The snakehead family originates from Asia and parts of Africa. The Northern Snakehead, which is invasive in the United States, originates from Southeast China and Korea. Snakeheads are apex predators, meaning that they stand at the top of the food chain and eat almost anything they can get in their mouth. Females can release anywhere from 1,300 to 15,000 eggs during a single spawn. They can spawn up to five times in a single year. They can survive in waters which range in temperature from 0 to 30 degrees Celsius. What makes them more threatening is that they can survive out of water for four days by breathing air with modified organs, even longer if they construct a muddy burrow.
The first invasive snakehead in the United States was discovered in Spiritwood Lake in California in 1997. The first established population of snakeheads was found in Crofton, Maryland in 2002. This population provided proof that snakeheads were able to invade and flourish in US waters. Since then juvenile and adult snakeheads have been found in the Schuylkill River in Pennsylvania, Lake Wylie in North Carolina, Meadow Lake in New York, and several other states in the eastern United States. When snakeheads enter a new body of water they tend to disrupt the food chain. Juvenile snakeheads compete for food with juveniles of native species. Adults also compete for resources with adults of native species and become so aggressive that they will also kill and eat them.

Their aggressive behavior, distinct appearance, and large size made snakeheads a popular aquarium fish, although due to their potential to invade natural ecosystems, they are illegal in over half of the United States, including Pennsylvania, Maryland, and New York. Irresponsibility was the main cause of their invasion into US waters. We as responsible aquarists must realize the impacts that snakeheads, and many other species of fish, may potentially have in the wild to prevent these species turning from pets to pests.
I hope that this blog was informative and illustrated the importance of keeping our pets in the aquarium. Check back for more invasive species blogs. Photo courtesy of the U.S. Geological Survey.
Thanks Brandon!
Until Next Time,
Dave
 That Fish Blog – Aquarium Advice and Information
That Fish Blog – Aquarium Advice and Information

 Welcome back Patty Little to That Fish Blog!
Welcome back Patty Little to That Fish Blog! and Copepods; shrimp-like crustaceans that dwell in the substrate and rocks. Because of the thousands of species contained within these groups in Class Crustacea, I am not going into detail about the taxonomy of these organisms, but here are some basic facts about these tiny crustaceans.
and Copepods; shrimp-like crustaceans that dwell in the substrate and rocks. Because of the thousands of species contained within these groups in Class Crustacea, I am not going into detail about the taxonomy of these organisms, but here are some basic facts about these tiny crustaceans. These creatures eat phytoplankton (tiny plants and algae that also help make up plankton), small microzooplankton (the division of zooplankton that are smaller than 200 microns, or 1/127th of an inch in size), and detritus.
These creatures eat phytoplankton (tiny plants and algae that also help make up plankton), small microzooplankton (the division of zooplankton that are smaller than 200 microns, or 1/127th of an inch in size), and detritus.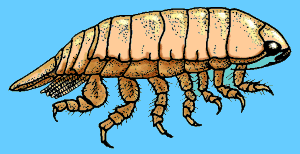 Another critter that may be seen is the isopod. Also called pill bugs, fish lice and rolly-pollies, these animals are found in all parts of the marine environment. Most isopods are free living and harmless, feeding on detritus and algaes, however, some are predatory, or parasitic, and dangerous to other reef aquarium animals.
Another critter that may be seen is the isopod. Also called pill bugs, fish lice and rolly-pollies, these animals are found in all parts of the marine environment. Most isopods are free living and harmless, feeding on detritus and algaes, however, some are predatory, or parasitic, and dangerous to other reef aquarium animals.